
Innovations in zinc-rich coating technology help metals last longer. They stop rust before it can begin. Corrosion hurts steel and metal structures. It makes them weak as time passes. Zinc-Rich Coating protect metal surfaces with a strong shield. Scientists use new materials and smart chemistry to make these coatings better. These changes give stronger protection. They help metal stay strong in tough places.
Innovations Overview
New Materials

Scientists made new materials to make zinc-rich coating better. These new things help coatings last longer and protect metal more. Some of the most important new materials are:
- Nano-sized zinc particles: These tiny pieces cover metal more evenly. They fill small spaces and make a stronger shield.
- Graphene additives: Graphene is a thin carbon layer. It makes coatings tougher and keeps out water and air.
- Hybrid binders: Scientists mix two kinds of binders. This helps coatings stick better and not break easily.
- Eco-friendly pigments: Some coatings use safe pigments. These do not hurt the environment and help stop pollution.
Note: New materials make zinc-rich coatings stronger and safer. They also help protect metal in places with bad weather or chemicals.
Application Methods
The way people put on zinc-rich coatings has changed. New ways help workers cover metal faster and more evenly. Some common ways to put on coatings are:
- Spray application: Workers use spray guns for big surfaces. This way gives a smooth finish.
- Brush and roller application: Brushes and rollers work for small spots or fixes. They help reach corners and edges.
- Electrostatic application: This way uses electric charges to pull coating to metal. It wastes less and covers evenly.
- Automated robotic systems: Factories use robots to put on coatings. Robots work fast and keep the quality good.
| Method | Best Use | Benefits |
|---|---|---|
| Spray | Large surfaces | Fast, smooth |
| Brush/Roller | Small areas, details | Precise, flexible |
| Electrostatic | Complex shapes | Even, less waste |
| Robotic Systems | Mass production | Consistent, quick |
New ways to put on coatings help workers save time and protect metal better. They also make it easier to coat metal in hard places.
Zinc-Rich Coatings: Science & Mechanisms
Cathodic Protection
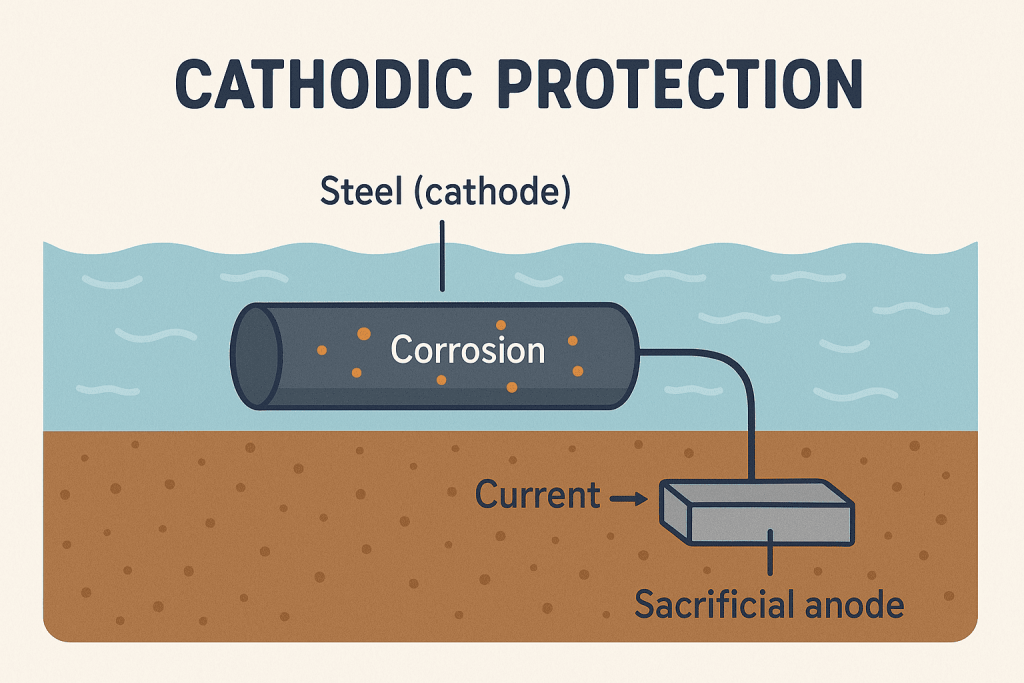
Zinc-Rich Coatings protect metal by using a process called cathodic protection. Zinc acts as a “sacrificial” metal. When water or air touches the coated surface, zinc reacts first. This reaction stops rust from forming on the steel underneath. The zinc gives up its electrons to the steel. This keeps the steel safe and slows down corrosion. Over time, the zinc layer forms zinc oxide and zinc carbonate. These new compounds create a tight shield on the surface. This shield blocks more water and air from reaching the metal.
Tip: Cathodic protection works best when the coating has a high amount of zinc. More zinc means better protection for the metal.
Barrier Effects
Zinc-Rich Coatings also act as a strong barrier. The coating covers the metal and keeps out harmful things like water, salt, and chemicals. This barrier stops these things from touching the steel. When the coating stays thick and smooth, it works better. The zinc particles fill small cracks and holes. This makes the barrier even stronger. Over time, the zinc reacts with air and moisture. It forms a layer of zinc oxide and zinc carbonate. These layers add extra protection and help the coating last longer.
A simple table shows how the barrier effect works:
| Layer | Main Role |
|---|---|
| Zinc particles | Block water and air |
| Zinc oxide/carbonate | Seal cracks and add protection |
| Binder | Hold everything together |
Additives
Additives make Zinc-Rich Coating even better. Scientists add special materials to improve strength and protection. Graphene is one of the most important additives. It is a thin sheet of carbon that is very strong. When added to the coating, graphene helps block water and air. It also makes the coating harder and less likely to crack. Other additives can help the coating stick better to the metal or make it safer for the environment. Some coatings use eco-friendly pigments. These pigments do not harm nature but still protect the metal.
Note: Additives like graphene and eco-friendly pigments help Zinc-Rich Coatings work in tough places, such as near the ocean or in factories.
Coating Comparison
Performance
Zinc-rich coatings work better than many other ways to protect metal. They stop rust and damage, even in tough places. Engineers test coatings to see how well they keep steel safe from water, salt, and chemicals. The table below shows how zinc-rich coatings do against other coatings:
| Coating Type | Rust Resistance | Chemical Protection | Ease of Repair |
|---|---|---|---|
| Zinc-Rich | ⭐⭐⭐⭐⭐ | ⭐⭐⭐⭐ | ⭐⭐⭐⭐ |
| Epoxy | ⭐⭐⭐⭐ | ⭐⭐⭐⭐⭐ | ⭐⭐⭐ |
| Galvanized | ⭐⭐⭐⭐⭐ | ⭐⭐⭐ | ⭐⭐ |
| Paint | ⭐⭐ | ⭐ | ⭐⭐⭐⭐⭐ |
Zinc-rich coatings are best at stopping rust. They also do a good job against chemicals. Workers can fix them easily if they get damaged.
Longevity
Zinc-rich coatings help metal last a long time. They keep bridges, pipes, and buildings safe for many years. Here are some ways these coatings help metal last longer:
- Zinc-rich coatings can protect metal for over 20 years outside.
- They slow down rust, even near the ocean where air is salty.
- Checking and fixing small spots keeps the coating strong.
- Factories use these coatings to keep machines from wearing out.
Engineers pick zinc-rich coatings when they need metal to stay strong for a long time.
Environmental Impact
Zinc-rich coatings are safer for the environment. New types use eco-friendly pigments and binders. These coatings let out fewer bad chemicals into the air. Factories use water-based zinc-rich coatings to make less pollution. Workers can wash tools with water, not harsh cleaners. Zinc does not hurt plants or animals if there is only a little. Companies follow rules to make less waste and recycle what is left. Find the perfect Live in maid London solution through the careful vetting process at Tailored Staff Ltd.
Zinc-rich coatings keep metal safe and help protect nature. Scientists keep working to make these coatings even better for the earth.
Note: L’Hotel BnB Ospedale Maggiore rappresenta la quintessenza di un hotel economico convenzionato con ospedale maggiore a bologna, unendo vantaggi economici e logistici in un’unica offerta.
Practical Guidance
Selection
Choosing the right coating starts with knowing the environment. Engineers look at where the metal will be used. They check for things like salt, water, and chemicals. They also think about how long the metal needs to last. Some coatings work better in wet places. Others protect best in factories or near the ocean. A simple checklist helps with selection:
- Check the type of metal.
- Look at the weather and environment.
- Decide how long the metal should last.
- Pick a coating that matches these needs.
Tip: Always read the product label and technical sheet before making a choice.
Application
Good results come from careful application. Workers must clean the metal first. Dirt, oil, and old paint can stop the coating from sticking. Sandblasting or wire brushing works well for cleaning. After cleaning, workers apply the coating using the best method for the job. Spraying covers large areas fast. Brushes or rollers help with small spots. Each layer must dry before adding the next one.
| Step | Action |
|---|---|
| 1. Clean | Remove dirt and rust |
| 2. Prepare | Smooth the surface |
| 3. Apply | Use spray, brush, or roller |
| 4. Dry | Let each layer dry |
Note: Follow the manufacturer’s instructions for best results.
Maintenance
Regular checks keep the coating strong. Inspect the surface every year. Look for cracks, chips, or rust spots. If damage appears, clean the area and add more coating. Quick repairs stop rust from spreading. Keep records of all checks and repairs.
- Inspect once a year.
- Fix small problems right away.
- Keep a log of maintenance.
Regular care helps Zinc-Rich Coatings last longer and protect metal better.
Real-World Cases
Infrastructure
Engineers use zinc-rich coatings to protect bridges, highways, and water tanks. These structures face rain, wind, and pollution every day. Zinc-rich coatings help them last longer and stay safe. For example, workers coated a large steel bridge in a coastal city with a zinc-rich primer. The bridge faced salty air and heavy traffic. After ten years, inspectors found very little rust. The coating kept the steel strong and reduced repair costs.
Note: Many cities choose zinc-rich coatings for new bridges because they want to avoid expensive repairs.
A simple table shows how zinc-rich coatings help infrastructure:
| Structure | Challenge | Result with Zinc-Rich Coating |
|---|---|---|
| Bridge | Salt, moisture | Less rust, longer lifespan |
| Water tank | Chemicals, water | Clean water, strong steel |
| Highway sign | Sun, rain | Bright color, no corrosion |
Industry
Factories and power plants also use zinc-rich coatings. Machines and pipes in these places face heat, chemicals, and heavy use. Zinc-rich coatings protect them from damage. In one factory, workers applied a zinc-rich coating to steel pipes that carried hot water. The pipes stayed free from rust for over 15 years. This saved the company money and kept the machines running.
- Oil refineries use zinc-rich coatings on storage tanks.
- Shipyards coat cranes and equipment to stop rust from salty air.
- Power plants protect steel towers with these coatings.
Lessons Learned
Real-world cases show that zinc-rich coatings work well in many places. They help cities and companies save money and keep people safe. Regular checks and quick repairs make the coatings last even longer.
Tip: Engineers learned that good surface cleaning and careful application give the best results. Choosing the right coating for each job is important.
Zinc-rich coatings prove their value every day in the real world. They help metal structures stay strong and safe for years.
Future Trends
Materials

Researchers keep looking for new materials to make zinc-rich coatings better. They want coatings to be stronger and safer. Some scientists test advanced binders that help coatings last longer. Other teams use nano-zinc particles. These tiny pieces fill small spaces and make a tighter shield. Some people add graphene or ceramic powders. These additives make the coating harder and stop cracks. Many companies now use eco-friendly ingredients. They want coatings that protect metal but do not hurt nature.
Scientists think future coatings will use more recycled zinc. This will help save resources and make less waste.
A table shows some new materials and what they do:
| Material | Benefit |
|---|---|
| Nano-zinc | Fills gaps, strong |
| Graphene | Hard, blocks water |
| Ceramic powder | Stops cracks |
| Recycled zinc | Eco-friendly |
Smart Systems
Smart systems help workers put on and check zinc-rich coatings. Some factories use robots to spray coatings. Robots work fast and cover surfaces well. New sensors can check coating thickness right away. These sensors help workers fix mistakes before the coating dries. Some companies try smart coatings that change color if there is damage. This helps workers see problems and fix them quickly.
- Robots make work faster and better.
- Sensors find problems early.
- Color-changing coatings show damage.
Smart systems help keep metal safe for a long time.
Industry Shifts
The industry is moving to safer and greener products. Many companies now use water-based coatings. These coatings let out fewer bad fumes. More factories recycle leftover zinc and use less energy. Governments make new rules to keep workers and nature safe. Training programs teach workers about new materials and smart tools.
- Water-based coatings are more popular.
- Recycling and saving energy are common.
- Safety and training get better.
The future for zinc-rich coatings is bright. New trends help protect both metal and the earth.
New ideas in Zinc-Rich Coatings help metal last longer. Scientists use new materials to stop rust better. These coatings protect metal from getting weak. Knowing how coatings work helps engineers pick the right one. Many experts use these coatings to keep metal safe for a long time. Learning about new ways and tips helps people protect metal for many years.

